Using "Molecular Glues" to Hijack the Body’s Garbage Disposal System

Proteins are the stuff of life. They comprise the hormones, enzymes and many other molecules that are essential to our body’s functioning. But sometimes these loyal workhorses can go rogue, leading to cancer, autoimmune diseases and a variety of neurological conditions.
Scientists who develop drugs are always looking for new ways to target these disease-related proteins — either to shut down their activities or to turn them up — depending on the circumstances. But a novel new approach to drug discovery called targeted protein degradation seeks to outright destroy these troublesome proteins. By co-opting the body's own natural "garbage disposal system," called the proteasome, this class of medicines marks these rogue players for destruction and sends them off for recycling within the cell.
This approach shows great promise for a class of proteins related to cancer, known as transcription factors, which have historically been deemed extremely difficult to target with a medicine.
“We can really explore an “undruggable” target, one that we couldn’t touch before,” says Andrea Weston, an Associate Research Fellow in the Primary Pharmacology Group at Pfizer’s Groton, Connecticut research site. “It’s a totally different drug paradigm because it opens up so many possibilities.”
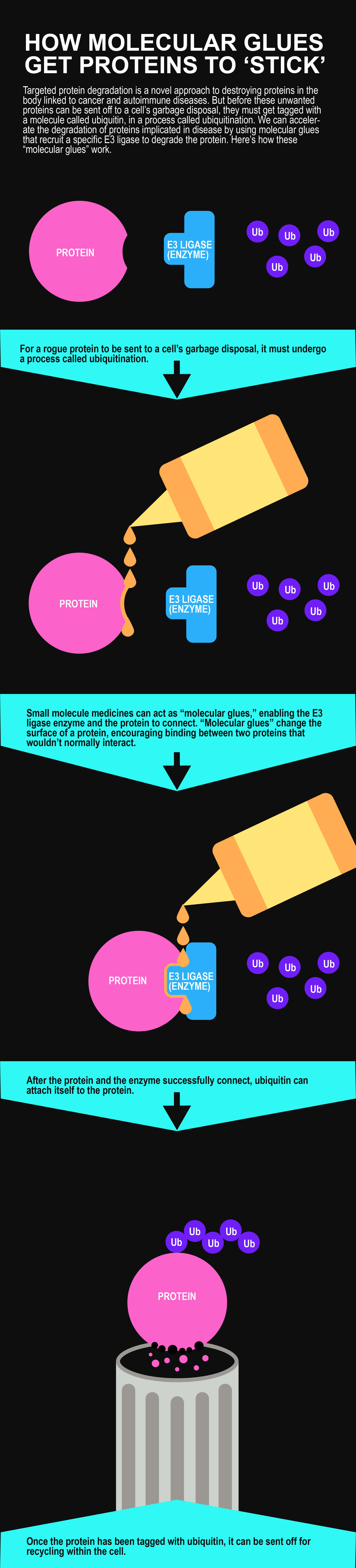
Getting proteins to “stick”
Before a rogue protein can be sent off to a cell’s garbage disposal it first must be tagged by a molecule called ubiquitin in a process known as ubiquitination. The challenge with this critical step is that not all proteins bind easily to the enzyme that is responsible for attaching ubiquitin. To overcome this hurdle, scientists are trying to develop small molecule medicines that act as "molecular glues" in an effort to help the enzymes "stick" better to the target proteins. Molecular glues are described as a type of molecule that encourages two proteins to come together that normally wouldn't interact. Known glue molecules achieve this by changing the surface of their target proteins.
The “molecular glue” can be used in two ways: it can bind to the enzyme, known as an E3 ligase, or to the target protein. In either scenario, the protein binding sites are slightly altered making them more favorable for matching up and kick-starting the ubiquitination process. “We’re creating novel protein-binding situations in the lab and those tests have shown promise in hijacking this natural system to degrade proteins,” says Regis Doyonnas, an Associate Research Fellow who works with Weston in the Primary Pharmacology Group.
Now that researchers have identified the protein degradation process they hope to kick-start, finding the right “glue” to support that degradation will be the key challenge. “We’re starting with a small molecule library of millions of compounds,” says Weston. "We're looking for such a needle in the haystack."
